Prognostic Significance of the Myelodysplastic Syndrome-Specific Comorbidity Index (MDS-CI) in Patients with Myelofibrosis: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Data Collection
2.3. Molecular Profiling
2.4. Prognostic Scoring Systems
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Prevalence of Comorbidities at Diagnosis and Hematological Parameters and Markers of Systemic Inflammation in Patients with and without Comorbidities
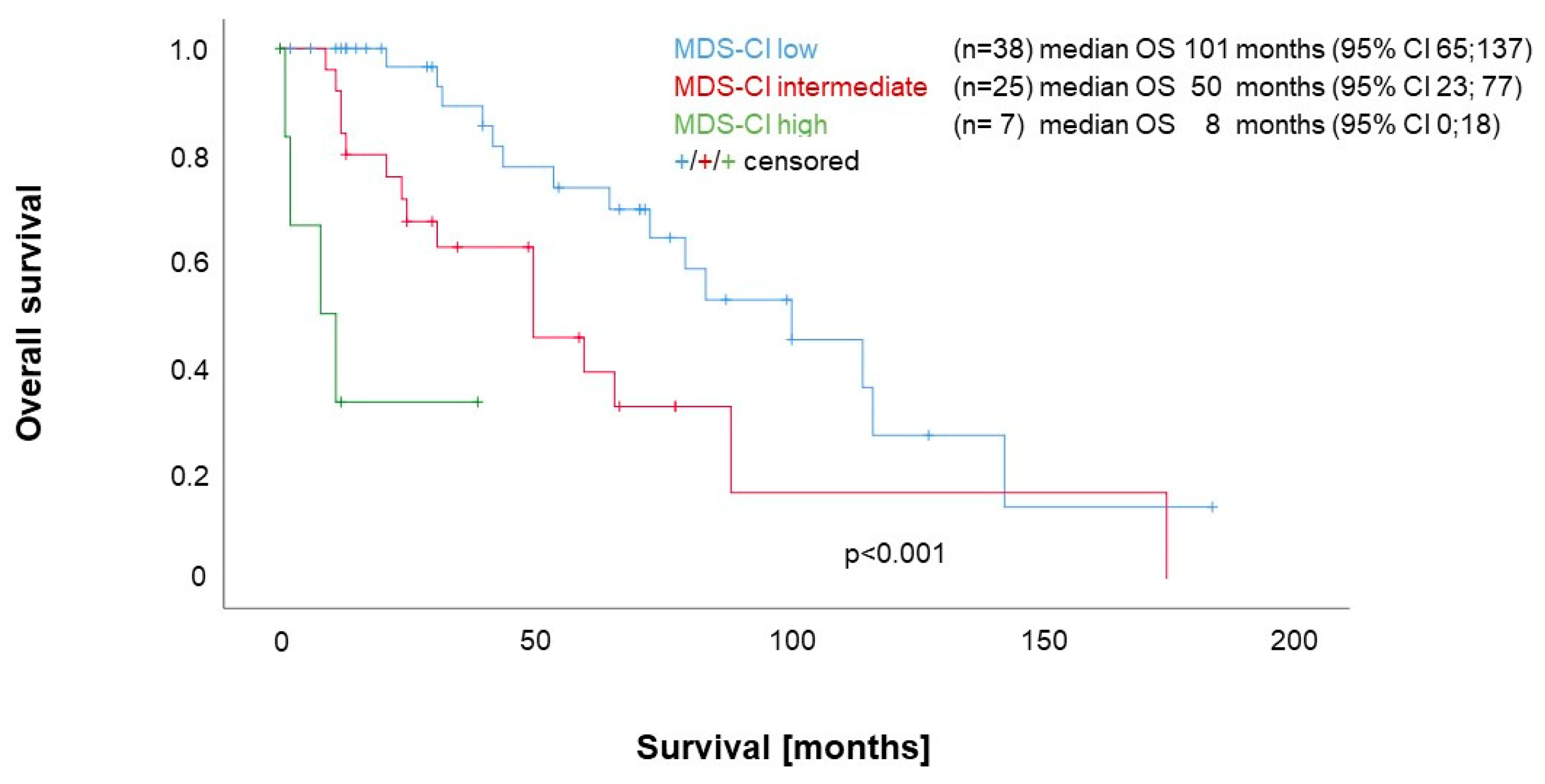
3.3. Impact of Comorbidities according to the MDS-CI on Survival
3.4. Impact of the MDS-CI in the Context of the DIPSS
3.5. Impact of the MDS-CI in the Context of the MIPSS70
3.6. Additional Prognostic Value of the MDS-CI and Model Performance
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Passamonti, F.; Mora, B. Myelofibrosis. Blood 2023, 141, 1954–1970. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, G.; McMullin, M.F.; Mills, K. Molecular Pathogenesis of the Myeloproliferative Neoplasms. J. Hematol. Oncol. 2021, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Luque Paz, D.; Kralovics, R.; Skoda, R.C. Genetic Basis and Molecular Profiling in Myeloproliferative Neoplasms. Blood 2023, 141, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C.; Bjørn, M.E. MPNs as Inflammatory Diseases: The Evidence, Consequences, and Perspectives. Mediat. Inflamm. 2015, 2015, 102476. [Google Scholar] [CrossRef]
- Fisher, D.A.C.; Fowles, J.S.; Zhou, A.; Oh, S.T. Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms. Front. Immunol. 2021, 12, 683401. [Google Scholar] [CrossRef]
- Cervantes, F.; Dupriez, B.; Pereira, A.; Passamonti, F.; Reilly, J.T.; Morra, E.; Vannucchi, A.M.; Mesa, R.A.; Demory, J.-L.; Barosi, G.; et al. New Prognostic Scoring System for Primary Myelofibrosis Based on a Study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009, 113, 2895–2901. [Google Scholar] [CrossRef]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Pereira, A.; Guglielmelli, P.; Pungolino, E.; Caramella, M.; Maffioli, M.; et al. A Dynamic Prognostic Model to Predict Survival in Primary Myelofibrosis: A Study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood 2010, 115, 1703–1708. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Guglielmelli, P. Molecular Prognostication in Ph-Negative MPNs in 2022. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 225–234. [Google Scholar] [CrossRef]
- Duminuco, A.; Nardo, A.; Giuffrida, G.; Leotta, S.; Markovic, U.; Giallongo, C.; Tibullo, D.; Romano, A.; Di Raimondo, F.; Palumbo, G.A. Myelofibrosis and Survival Prognostic Models: A Journey between Past and Future. J. Clin. Med. 2023, 12, 2188. [Google Scholar] [CrossRef]
- García-Fortes, M.; Hernández-Boluda, J.C.; Álvarez-Larrán, A.; Raya, J.M.; Angona, A.; Estrada, N.; Fox, L.; Cuevas, B.; García-Hernández, M.C.; Gómez-Casares, M.T.; et al. Impact of Individual Comorbidities on Survival of Patients with Myelofibrosis. Cancers 2022, 14, 2331. [Google Scholar] [CrossRef]
- Lucijanic, M.; Galusic, D.; Krecak, I.; Sedinic, M.; Holik, H.; Perisa, V.; Moric Peric, M.; Zekanovic, I.; Stoos-Veic, T.; Kusec, R. Reduced Renal Function Strongly Affects Survival and Thrombosis in Patients with Myelofibrosis. Ann. Hematol. 2020, 99, 2779–2785. [Google Scholar] [CrossRef] [PubMed]
- Sochacki, A.L.; Bejan, C.A.; Zhao, S.; Patel, A.; Kishtagari, A.; Spaulding, T.P.; Silver, A.J.; Stockton, S.S.; Pugh, K.; Dorand, R.D.; et al. Patient-Specific Comorbidities as Prognostic Variables for Survival in Myelofibrosis. Blood Adv. 2023, 7, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.; Panzarella, T.; McNamara, C.J.; Lau, A.; Schimmer, A.D.; Schuh, A.C.; Sibai, H.; Maze, D.; Yee, K.W.L.; Devlin, R.; et al. Distribution and Impact of Comorbidities on Survival and Leukemic Transformation in Myeloproliferative Neoplasm-Associated Myelofibrosis: A Retrospective Cohort Study. Clin. Lymphoma Myeloma Leuk. 2017, 17, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Bartoletti, D.; Bonifacio, M.; Palumbo, G.A.; Polverelli, N.; Abruzzese, E.; Bergamaschi, M.; Tieghi, A.; Tiribelli, M.; Iurlo, A.; et al. Impact of Comorbidities and Body Mass Index in Patients with Myelofibrosis Treated with Ruxolitinib. Ann. Hematol. 2019, 98, 889–896. [Google Scholar] [CrossRef]
- Lekovic, D.; Gotic, M.; Perunicic-Jovanovic, M.; Vidovic, A.; Bogdanovic, A.; Jankovic, G.; Cokic, V.; Milic, N. Contribution of Comorbidities and Grade of Bone Marrow Fibrosis to the Prognosis of Survival in Patients with Primary Myelofibrosis. Med. Oncol. 2014, 31, 869. [Google Scholar] [CrossRef]
- Newberry, K.J.; Naqvi, K.; Nguyen, K.T.; Cardenas-Turanzas, M.; Florencia Tanaka, M.; Pierce, S.; Verstovsek, S. Comorbidities Predict Worse Prognosis in Patients with Primary Myelofibrosis: Comorbidities in Primary Myelofibrosis/Newberry et Al. Cancer 2014, 120, 2996–3002. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Malcovati, L.; Strupp, C.; Ambaglio, I.; Kuendgen, A.; Zipperer, E.; Travaglino, E.; Invernizzi, R.; Pascutto, C.; Lazzarino, M.; et al. Risk Stratification Based on Both Disease Status and Extra-Hematologic Comorbidities in Patients with Myelodysplastic Syndrome. Haematologica 2011, 96, 441–449. [Google Scholar] [CrossRef]
- Zipperer, E.; Tanha, N.; Strupp, C.; Kündgen, A.; Nachtkamp, K.; Neukirchen, J.; Hildebrandt, B.; Haas, R.; Gattermann, N.; Germing, U. The Myelodysplastic Syndrome-Comorbidity Index Provides Additional Prognostic Information on Patients Stratified According to the Revised International Prognostic Scoring System. Haematologica 2014, 99, e31–e32. [Google Scholar] [CrossRef]
- Marcellino, B.K.; Verstovsek, S.; Mascarenhas, J. The Myelodepletive Phenotype in Myelofibrosis: Clinical Relevance and Therapeutic Implication. Clin. Lymphoma Myeloma Leuk. 2020, 20, 415–421. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 310–318. [Google Scholar] [CrossRef]
- Lee, E.S.; Koh, H.L.; Ho, E.Q.-Y.; Teo, S.H.; Wong, F.Y.; Ryan, B.L.; Fortin, M.; Stewart, M. Systematic Review on the Instruments Used for Measuring the Association of the Level of Multimorbidity and Clinically Important Outcomes. BMJ Open 2021, 11, e041219. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Boluda, J.-C.; Pereira, A.; Alvarez-Larran, A.; Martín, A.-A.; Benzaquen, A.; Aguirre, L.; Mora, E.; González, P.; Mora, J.; Dorado, N.; et al. Predicting Survival after Allogeneic Hematopoietic Cell Transplantation in Myelofibrosis: Performance of the Myelofibrosis Transplant Scoring System (MTSS) and Development of a New Prognostic Model. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2020, 26, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Medina, A.A.; Baranwal, A.; Johnson, I.M.; Kharfan-Dabaja, M.A.; Murthy, H.; Palmer, J.M.; Sproat, L.; Mangaonkar, A.; Shah, M.V.; Hogan, W.J.; et al. Comparison of Pretransplantation Prediction Models for Nonrelapse Mortality in Patients with Myelofibrosis Undergoing Allogeneic Stem Cell Transplantation. Transplant. Cell. Ther. 2023, 29, 360.e1–360.e8. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Thomas, J.W.; Ruan, G.; Hyun, M.C.; Bansal, R.; McLaughlin, N.; Pardanani, A.; Gangat, N.; Go, R.S.; Szuber, N.; et al. A Population-based Study of Outcomes in Polycythemia Vera, Essential Thrombocythemia, and Primary Myelofibrosis in the United States from 2001 to 2015: Comparison with Data from a Mayo Clinic Single Institutional Series. Am. J. Hematol. 2021, 96, E464–E468. [Google Scholar] [CrossRef]
- Dores, G.M.; Curtis, R.E.; Linet, M.S.; Morton, L.M. Cause-Specific Mortality Following Polycythemia Vera, Essential Thrombocythemia, and Primary Myelofibrosis in the U.S. Population, 2001-2017. Am. J. Hematol. 2021, 96, E451–E454. [Google Scholar] [CrossRef]
- Gecht, J.; Tsoukakis, I.; Kricheldorf, K.; Stegelmann, F.; Klausmann, M.; Griesshammer, M.; Schulz, H.; Hollburg, W.; Göthert, J.R.; Sockel, K.; et al. Kidney Dysfunction Is Associated with Thrombosis and Disease Severity in Myeloproliferative Neoplasms: Implications from the German Study Group for MPN Bioregistry. Cancers 2021, 13, 4086. [Google Scholar] [CrossRef]
- Lucijanic, M.; Krecak, I.; Kusec, R. Renal Disease Associated with Chronic Myeloproliferative Neoplasms. Expert Rev. Hematol. 2022, 15, 93–96. [Google Scholar] [CrossRef]
- Aspite, S.; Schepis, F.; Roccarina, D.; Gitto, S.; Citone, M.; Di Bonaventura, C.; Bianchini, M.; Arena, U.; Vannucchi, A.M.; Guglielmelli, P.; et al. Portosystemic Shunt Is an Effective Treatment for Complications of Portal Hypertension in Hepatic Myeloid Metaplasia and Improves Nutritional Status. Liver Int. Off. J. Int. Assoc. Study Liver 2022, 42, 419–424. [Google Scholar] [CrossRef]
- Liu, A.; Naymagon, L.; Tremblay, D. Splanchnic Vein Thrombosis in Myeloproliferative Neoplasms: Treatment Considerations and Unmet Needs. Cancers 2023, 15, 11. [Google Scholar] [CrossRef]
- Isfort, S.; Kaifie, A.; Bennemann, K.; Jost, E.; Panse, J.; Bruemmendorf, T.H.; Koschmieder, S. Comorbidity Scales in Patients with Myeloproliferative Neoplasms: Which One Fits Best? Blood 2014, 124, 1828. [Google Scholar] [CrossRef]
- Fabritz, L.; Guasch, E.; Antoniades, C.; Bardinet, I.; Benninger, G.; Betts, T.R.; Brand, E.; Breithardt, G.; Bucklar-Suchankova, G.; Camm, A.J.; et al. Expert Consensus Document: Defining the Major Health Modifiers Causing Atrial Fibrillation: A Roadmap to Underpin Personalized Prevention and Treatment. Nat. Rev. Cardiol. 2016, 13, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Farcomeni, A. Association Between Peripheral Artery Disease and Incident Risk of Atrial Fibrillation: Strong Evidence Coming From Population-Based Cohort Studies. J. Am. Heart Assoc. 2018, 7, e009126. [Google Scholar] [CrossRef] [PubMed]
- Junejo, R.T.; Lip, G.Y.H.; Fisher, J.P. Cerebrovascular Dysfunction in Atrial Fibrillation. Front. Physiol. 2020, 11, 1066. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Sacuiu, S.; Wetterberg, H.; Najar, J.; Guo, X.; Kern, S.; Zettergren, A.; Shams, S.; Pereira, J.B.; Wahlund, L.-O.; et al. Atrial Fibrillation, Stroke, and Silent Cerebrovascular Disease: A Population-Based MRI Study. Neurology 2021, 97, e1608–e1619. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C. Perspectives on the Impact of JAK-Inhibitor Therapy upon Inflammation-Mediated Comorbidities in Myelofibrosis and Related Neoplasms. Expert Rev. Hematol. 2014, 7, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK-STAT Pathway: An Emerging Target for Cardiovascular Disease in Rheumatoid Arthritis and Myeloproliferative Neoplasms. Eur. Heart J. 2021, 42, 4389–4400. [Google Scholar] [CrossRef]
- Passamonti, F.; Giorgino, T.; Mora, B.; Guglielmelli, P.; Rumi, E.; Maffioli, M.; Rambaldi, A.; Caramella, M.; Komrokji, R.; Gotlib, J.; et al. A Clinical-Molecular Prognostic Model to Predict Survival in Patients with Post Polycythemia Vera and Post Essential Thrombocythemia Myelofibrosis. Leukemia 2017, 31, 2726–2731. [Google Scholar] [CrossRef]
- Tefferi, A.; Nicolosi, M.; Mudireddy, M.; Lasho, T.L.; Gangat, N.; Begna, K.H.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A. Revised Cytogenetic Risk Stratification in Primary Myelofibrosis: Analysis Based on 1002 Informative Patients. Leukemia 2018, 32, 1189–1199. [Google Scholar] [CrossRef]


| Whole Population | MDS-CI 0 | MDS-C1 ≥1 | p-Value | |
|---|---|---|---|---|
| n | 70 | 38 | 32 | |
| Age (years), median (IQR) | 73 (63–78) | 70 (59–76) | 77 (67–80) | 0.005 |
| Female n (%) | 33/70 (47.1) | 18/38 (47.4) | 15/32 (46.9) | 1.00 |
| Bone marrow fibrosis grade 2, n (%) | 49/70 (70) | 24/38 (63.2) | 25/32 (78.1) | |
| Bone marrow fibrosis grade 3, n (%) | 21/70 (30) | 14/38 (36.8) | 7/32 (21.9) | 0.200 |
| Hemoglobin (g/L), median (IQR) | 110 (88–123) | 114 (99–124) | 99 (83–121) | 0.128 |
| Platelet count (×109/L), median (IQR) | 411 (199–683) | 481 (197–697) | 391 (222–648) | 0.700 |
| Leukocytes (×109/L), median (IQR) | 9.3 (6.5–16.0) | 7.7 (6.4–13.4) | 11.4 (7.0–21.0) | 0.067 |
| Neutrophils (×109/L), median (IQR) | 6.6 (3.9–12.8) | 6.1 (3.8–10.5) | 7.7 (4.7–15.1) | 0.166 |
| Monocytes (×109/L), median (IQR) | 0.57 (0.35–0.84) | 0.64 (0.41–0.83) | 0.46 (0.27–1.09) | 0.489 |
| Lymphocytes (×109/L), median (IQR) | 1.5 (1.0–2.2) | 1.5 (1.0–2.0) | 1.5 (1.0–2.3) | 0.781 |
| Blasts PB (%), Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.075 |
| Constitutional symptoms, n (%) | 33/70 (47.1) | 14/38 (36.8) | 19/32 (59.4) | 0.092 |
| LDH available (U/L), median (IQR) | 62/70 530 (355–686) | 33/38 525 (365–659) | 29/32 554 (327–789) | 0.672 |
| CRP available (mg/L), median (IQR) | 65/70 5 (2–12) | 33/38 3 (1–7) | 32/32 9 (5–29) | < 0.001 |
| Ferritin available (μg/L), median (IQR) | 49/70 151 (69–275) | 24/38 122 (55–176) | 25/32 210 (116–396) | 0.009 |
| Albumin available (g/L), median (IQR) | 55/70 39.9 (37.0–42.3) | 28/38 41.9 (37.9–42.9) | 27/32 38.2 (36.2–42.1) | 0.056 |
| Need for transfusion, n (%) | 23/70 (32.9) | 11/38 (28.9) | 12/32 (37.5) | 0.610 |
| Splenomegaly (clinical or imaging) | 56/70 (80) | 28/38 (73.7) | 28/32 (87.5) | 0.231 |
| BMI available (kg/m2), median (IQR) | 65/70 24.5 (21.2–28.0) | 33/38 23.2 (21.0–28.0) | 32/32 25.0 (21.2–28.1) | 0.512 |
| Multivariate 1 | Multivariate 2 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| MDS-CI * | ||||||
| Intermediate | 1.97 | 0.96; 4.03 | 0.065 | 1.63 | 0.65; 4.06 | 0.2961 |
| High | 14.64 | 4.42; 48.48 | <0.001 | 19.65 | 4.71; 81.95 | <0.001 |
| DIPSSdich ** | 6.08 | 2.35; 15.71 | 0.0002 | |||
| MIPSS70dich *** | 4.53 | 1.64; 12.53 | 0.0036 | |||
| Model | N | LL | df | AIC | BIC | LR Test p-Value | C-Index |
|---|---|---|---|---|---|---|---|
| DIPSSdich | 70 | −105.0 | 1 | 211.9 | 214.2 | 0.6999 | |
| DIPSSdich and MDS-CI | 70 | −98.7 | 3 | 0.0018 | 0.7814 | ||
| MIPSS70dich | 56 | −73.4 | 1 | 148.7 | 150.8 | 0.6515 | |
| MIPSS70dich and MDS-CI | 56 | −67.0 | 1 | 139.9 | 146.0 | 0.0017 | 0.7770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koster, K.-L.; Messerich, N.-M.; Volken, T.; Cogliatti, S.; Lehmann, T.; Graf, L.; Holbro, A.; Benz, R.; Demmer, I.; Jochum, W.; et al. Prognostic Significance of the Myelodysplastic Syndrome-Specific Comorbidity Index (MDS-CI) in Patients with Myelofibrosis: A Retrospective Study. Cancers 2023, 15, 4698. https://doi.org/10.3390/cancers15194698
Koster K-L, Messerich N-M, Volken T, Cogliatti S, Lehmann T, Graf L, Holbro A, Benz R, Demmer I, Jochum W, et al. Prognostic Significance of the Myelodysplastic Syndrome-Specific Comorbidity Index (MDS-CI) in Patients with Myelofibrosis: A Retrospective Study. Cancers. 2023; 15(19):4698. https://doi.org/10.3390/cancers15194698
Chicago/Turabian StyleKoster, Kira-Lee, Nora-Medea Messerich, Thomas Volken, Sergio Cogliatti, Thomas Lehmann, Lukas Graf, Andreas Holbro, Rudolf Benz, Izadora Demmer, Wolfram Jochum, and et al. 2023. "Prognostic Significance of the Myelodysplastic Syndrome-Specific Comorbidity Index (MDS-CI) in Patients with Myelofibrosis: A Retrospective Study" Cancers 15, no. 19: 4698. https://doi.org/10.3390/cancers15194698
APA StyleKoster, K.-L., Messerich, N.-M., Volken, T., Cogliatti, S., Lehmann, T., Graf, L., Holbro, A., Benz, R., Demmer, I., Jochum, W., Rao, T. N., & Silzle, T. (2023). Prognostic Significance of the Myelodysplastic Syndrome-Specific Comorbidity Index (MDS-CI) in Patients with Myelofibrosis: A Retrospective Study. Cancers, 15(19), 4698. https://doi.org/10.3390/cancers15194698






